BLOG
Updated October 11, 2023
On October 6, 2023, the DEA extended Telemedicine Flexibilities for Prescribing Controlled Substances for a second time.
Nearly one month after two full days of DEA organized listening sessions on September 12th and 13th, another temporary rule has been released extending Telemedicine Flexibilities for Prescribing Controlled Substances through December 31st, 2024. This will again, eliminate the requirement of an in-person visit before being prescribed medications that fall within the controlled substances umbrella. But it isn’t expected to last forever. The DEA is currently back to rule-writing, as they will likely share a new draft of a proposed rule early in 2024. There will be another comment period and the new rule will be announced before the temporary rule extension expires.
COVID-19 Telemedicine Flexibilities for Prescription of Controlled Medications
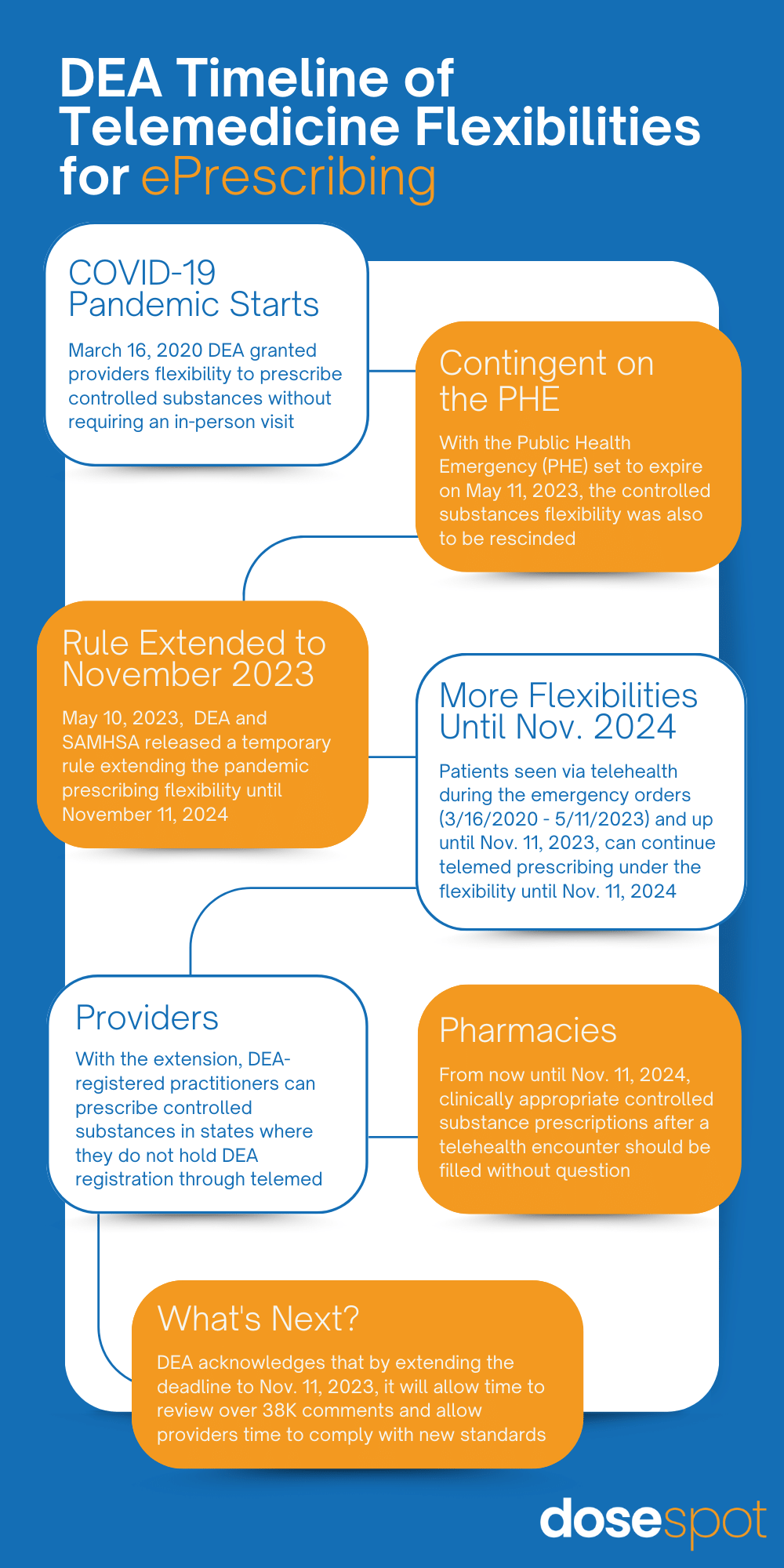
Throughout the COVID-19 pandemic, the Drug Enforcement Administration (DEA) granted providers the flexibility to prescribe clinically appropriate controlled substance medications without requiring an in-person visit, which was particularly crucial amidst the global shutdown. However, this allowance was contingent upon the Public Health Emergency (PHE) declared by the U.S. Health and Human Services (HHS) Secretary being in effect and was scheduled to be rescinded when it ended on May 11, 2023. With one day to spare on May 10, the DEA and the Substance Abuse and Mental Health Services Administration (SAMHSA) released a temporary rule extending the pandemic prescribing flexibility until November 11, 2023. Following this date, any patient seen by a provider via telehealth during the PHE (March 16, 2020 – May 11, 2023) and up until November 11, 2023 will be allowed to continue prescribing under the flexibility for an additional year until November 11, 2024. However, if DEA fails to finalize new rules by November 11, 2023, providers will have to follow pre-pandemic remote prescribing laws outlined in the Ryan Haight Act, which will require any new patient to be seen in-person before being eligible to receive a controlled substance prescription after a telehealth encounter.
Implications for Providers and Pharmacies
For Providers
Under this temporary rule, other DEA waivers were extended. DEA-registered practitioners are not obligated to acquire additional registration(s) with the DEA in the additional state(s) where dispensing (including prescribing and administering) takes place. Therefore, DEA-registered practitioners have the authority to prescribe controlled substances to patients in states where they do not hold DEA registration through telemedicine.
For Pharmacies
Pharmacies should adhere to the practice of dispensing medications based on valid prescriptions, regardless of whether they are issued through telehealth or through in-person visits. From now until November 11, 2024, clinically appropriate controlled substance prescriptions prescribed after a telehealth encounter should be filled without question.
What to Expect in the Coming Months
Prior to issuing this temporary rule, the DEA issued two proposed rules that would have imposed limitations on telehealth prescribing of controlled substances (CS) in cases where the practitioner and the patient have not had a prior in-person medical assessment and on the initiation of buprenorphine treatment via telemedicine, including audio-only encounters. Following stakeholders raising significant concerns about the proposed rules, as evidenced by the receipt of over 38,000 comments, DEA and SAMHSA decided to issue the temporary rule explained earlier.
The DEA acknowledges in the temporary rule that extending the deadline by six months until November 11, 2023, will provide the agency with additional time to review the numerous comments received. This extension aims to facilitate a seamless transition for patients and providers who have grown to rely on telemedicine encounters to get their medically necessary controlled substance prescriptions. Additionally, it will allow sufficient time for providers to comply with any new standards that may be established by the DEA and/or SAMHSA in any potential final rules or additional waiver extensions that may be issued before that deadline.
The DEA stated that it plans to release “one or more final rules” that build off their initial two proposed rules before November 11, 2023 that can be implemented by November 11, 2024; however, it is unclear at this time if the agency will meet this deadline. Any new rules that the DEA promulgates on the remote prescribing of controlled substances are expected to be permanent. The agency continues to emphasize that they are focused on allowing the practice of telemedicine in a manner that aligns with public health and safety objectives while ensuring effective measures against diversion.
Source: Faegre Drinker Consulting

